
Презентация по Химии "Хром" скачать смотреть бесплатно
Detailed information about the equation. Reaction conditions when applied H2SO4 + K2Cr2O7 + K2S. Reaction process H2SO4 + K2Cr2O7 + K2S. The result of the reaction H2SO4 + K2Cr2O7 + K2S.

Action Of Acidified K2Cr2O7 On Hydrogen Sulphide D and F Block Elements Chemistry Class 12
Click here:point_up_2:to get an answer to your question :writing_hand:balance the following equation by oxidation number methodk2cr2o7feso4h2so4rightarrow.
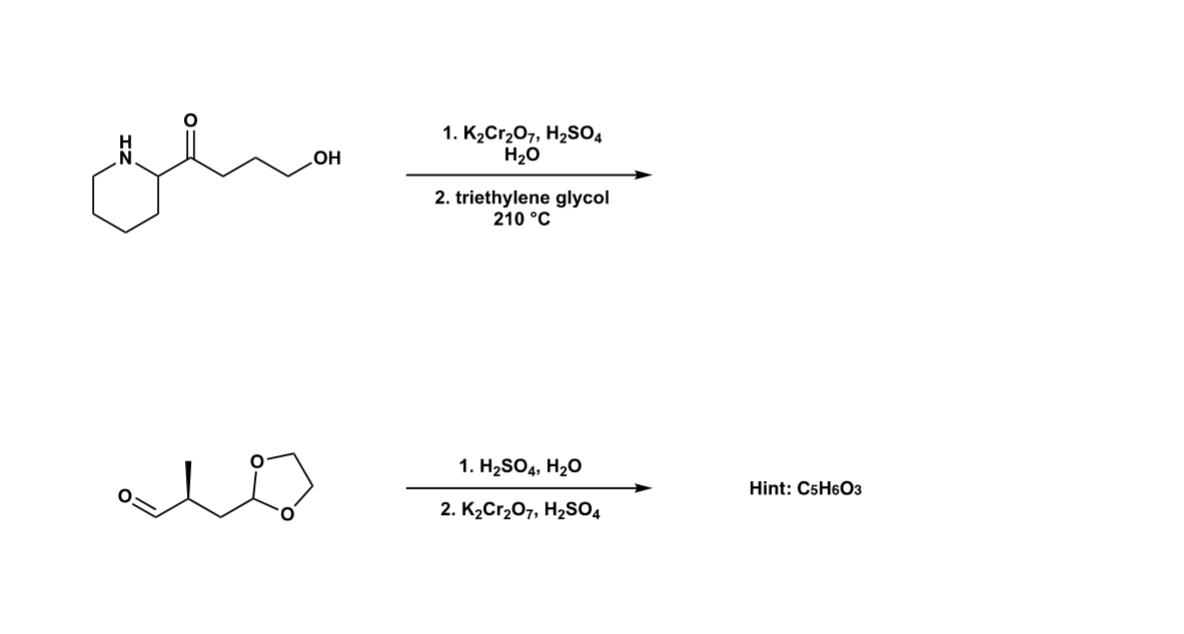
Solved 1. K2Cr2O7, H2SO4 H2O OH 2. triethylene glycol 210 °C
Detailed information about the equation. Reaction conditions when applied H2S + H2SO4 + K2Cr2O7. Reaction process H2S + H2SO4 + K2Cr2O7. The result of the reaction H2S + H2SO4 + K2Cr2O7.

please try to balance this equation K2Cr2O7 + H2SO4 > K2SO4 + Cr2(SO4)3 + H2O + O2 Brainly.in
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 0 K2Cr2O7 + 3 H2S + H2SO4 = 0 Cr2(SO4)3 + 0 K2SO4 + 4 S + 4 H2O. Reactants. Products.

Metode Ion Elektron K2Cr2O7 + H2S + H2SO4 YouTube
Solved and balanced chemical equation K2Cr2O7 + 3 H2O2 + 4 H2SO4 → Cr2(SO4)3 + 7 H2O + K2SO4 + 3 O2 with completed products. Application for completing products and balancing equations. Chemical Equations online! Submit. Advanced search. K 2 Cr 2 O 7 + 3 H 2 O 2 + 4 H 2 S O 4 → Cr 2 (S O 4) 3 + 7 H 2 O + K 2 S O 4 + 3 O 2. This.

how to balance h2o4 + h3s + 1 + h2o
Step 1. Write the skeleton equation. The molecular equation is. K2Cr2O7 + H2SO4 +SO2 → K2SO4 + Cr2(SO4)3 +H2O. Strip the equation of all common ions, also H+,OH- and H2O (these come back in during the balancing procedure). K+ + Cr2O2- 7 + H+ + SO2- 4 + SO2 → K+ + SO2- 4 +Cr3+ + SO2- 4 + H2O. The above equation simplifies to. Cr2O2- 7 +SO2.

a beakle filled with liquid and flasks containing orange, red and blue liquids
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 4 K2Cr2O7 + -3 H2S + 15 H2SO4 = 4 Cr2(SO4)3 + -8 S + 8 KHSO4 + 8 H2O. Reactants. Products.

Napisz reakcje redoks, wskaż reduktor, utleniacz i zapisz reakcje połówkowe K2Cr2O7 + H2S
Here's what I got. Start by writing the unbalanced chemical equation. Potassium dichromate, "K"_2"Cr"_2"O"_7, will react with hydrosulfuric acid, which is aqueous.

Ki reacts with h2so4 producing i2 and h2s the volume of 0.2 N h2so4 r askIITians
Balance Chemical Equation - Online Balancer. Word equation; 4 Potassium dichromate + 3 Sulfane + 13 Sulfuric acid = 4 Potassium sulfate + 4 Chromium(III) sulfate + 16 Water
K2Cr2O7 YouTube
Balance the chemical equation k2cr2o7+h2so4+so2=k2so4+cr2(so4)3+H2O Balance the chemical reaction K2Cr2O7+H2SO4+SO2=K2SO4+Cr2(SO4)3+H2O Potassium dicro.

K2Cr2O7 + H2S + H2SO4 =S +K2SO4 + Cr2(SO4)3 + H2O is a very common reaction. The chemistry
H2SO4 + K2CrO4 → H2O + K2Cr2O7 + K2SO4 | Equation Balance. Color some common substances.

Используя метод инноэлектронного баланса,расставьте коэф. в окислитвосст. реакциях.Определите
About K2Cr2O7 H2S H2SO4. Potassium dichromate (K2Cr2O7) is a common chemical inorganic reagent most commonly used as an oxidizing agent in various laboratories. It's a crystalline ionic strong, very bright, red-orange. Among chemists, Potassium Dichromate is very common in determining the unknown concentration of secondary standard substances solution.
Al + KMnO4 + H2SO4 = KHSO4 + Al2(SO4)3 + MnSO4 + H2O KNO3 + FeSO4 + H2SO4 = KHSO4 + Fe2(SO4)3
K2Cr2O7+H2SO4+SO2=K2SO4+Cr2(SO4)3+H2O balance the redox reaction by ion electron method or half reaction method. k2cr2o7+h2so4+so2=k2so4+cr2(so4)3+h2o.

Найдите сумму коэффициентов в правой части уравнения. H2S + H2SO4 + K2Cr2O7 = Cr2(SO4)3 + S
$$\ce{H2S + K2Cr2O7 + 4H2SO4 -> SO2 + Cr2(SO4)3 + K2SO4 + 5H2O}?\label{rxn:QR2}\tag{R2}$$ The reaction seems stoichiometrically plausible, yet likely requires highly concentrated oxidants. I know \eqref{rxn:QR1} happens at low concentration of $\ce{H2SO4}$. Maybe using highly concentrated $\ce{H2SO4}$ would do the work for \eqref{rxn:QR2}. What.

Uzgodnij współczynniki w następujących reakcjach Cr2O3 + KNO3 + KOH K2CrO4 + KNO2 + H2O
This reaction can be represented by the following equation: K2Cr2O7 + H2SO4 → H2CrO4 + K2SO4. Chromic acid is a strong oxidizing agent and plays a crucial role in the subsequent steps of the reaction. Oxidation of Sulfuric Acid: In the presence of chromic acid, sulfuric acid gets oxidized to form sulfur trioxide (SO3).

K2Cr2O7 + H2So4 / Which compound can be most easily oxidized... Clutch Prep It must have
The hydrogen atoms are already balanced, so we don't need to make any changes. The equation now looks like this: K2Cr2O7 + H2SO4 ---> K2SO4 + Cr2(SO4)3 + H2O + 2O2 Answer 3. Finally, we need to balance the number of potassium and sulfate ions. On the left side, we have 2 potassium ions from K2Cr2O7 and 1 sulfate ion from H2SO4.